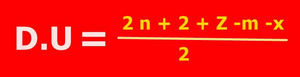Degree of Unsaturation ( D.U.)
Degree of Unsaturation ( D.U.) or Index of Hydrogen deficiency (I.H.D)
Degree of Unsaturation ( D.U.) is defined as total sum of total pi π bonds and no. of cycles (rings) in a compound.
DU from Structural formula.
Example 1.
Cyclobutadiene.
Cyclobutadiene has two pi bonds and one ring hence the D.U. of Cyclobutadiene is 2+1 = 3.
Example 2.
Napthalene
Napthalene has 5 pi bonds and two ring hence the D.U. of Napthalene is 5+2 = 7.
Example 3.
Pthallic acid
Pthallic Acid has 5 pi bonds and one ring hence the D.U. of Pthallic Acid is 5+1 = 6.
Example 4.
Bombykol
Bombykol has 2 pi bonds and no ring hence the D.U. of Bombykol is 2+0 =2.
Example 4.
Benzonitrile
Benzonitrile has 5 pi bonds and one ring hence the D.U. of Benzonitrile is 5+1 =6.
Example 5.
Cubane
Cubane has no pi bonds and five ring hence the D.U. of cubane is 0+5 =6.
DU from Molecular Formula.
If molecular formula of a compound is
C nH mCl xO yN z
then
D.U. is
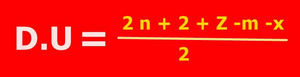
Example 1 For
C 10H 20
n =10 and m= 20 x =0 y =0 Z=0 hence D.U. = 1.
Example 2 For
C 10H 8
n =10 and m= 8 x =0 y =0 Z=0 hence D.U. = 14/2 =7.
Example 3 For
C 10H 8Cl 2
n =10 and m= 8 x =2 y =0 Z=0 hence D.U. = (22-8-2)/2 =6.